VAERS ANALYSIS
$529.00
The Drug Safety Signal Project: An Introduction to Pharmacovigilance Using VAERS
Uncover potential drug safety signals from real-world patient reports. This project is your ideal introduction to the critical field of pharmacovigilance, teaching you how to navigate and analyze one of the most important public health databases in the United States.
The Core Question We’ll Answer
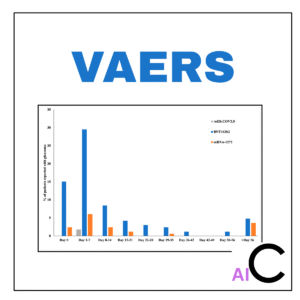
“For a specific drug or vaccine of your choice, what are the most frequently reported adverse events in the VAERS database, and how can we visualize these findings to identify potential safety signals for further investigation?”
The Challenge: Why This Project Matters
The Vaccine Adverse Event Reporting System (VAERS) is a rich but notoriously complex dataset. It contains hundreds of thousands of raw, unstructured reports directly from patients and healthcare providers. This data is messy, contains “noise,” and requires careful handling to extract meaningful insights. Simply downloading the files is easy; knowing how to clean, code, and interpret the data is a highly valuable skill. This project teaches you how to turn that raw data into actionable intelligence.
What You Will Learn (Your Personalized Skill Development)
- Pharmacovigilance Fundamentals: Understand the core principles of drug safety monitoring and the role of spontaneous reporting systems like VAERS.
- Handling Unstructured Data: Gain hands-on experience cleaning and structuring messy, real-world text data a critical skill for any data analyst.
- NLP for Signal Detection: Apply basic Natural Language Processing (NLP) techniques to perform frequency analysis on adverse event descriptions.
- Data Visualization for Safety: Learn to create compelling visualizations like word clouds and bar charts that make it easy to spot trends and potential signals in the data.
- Critical Interpretation: Master the most important part of working with VAERS: understanding its limitations and learning to interpret the findings correctly, distinguishing between correlation and causation.
What We Will Do For You (The “Co-Pilot” Process)
- Project Scoping: We’ll help you select an appropriate drug or vaccine of interest for the analysis.
- Data Extraction & Prep: We will download the latest VAERS data files, filter for your product, and perform the necessary data cleaning and preparation.
- Analysis & Visualization: Our experts will conduct the frequency analysis and generate a series of visualizations to highlight the key findings.
- The Co-Pilot Session: We will walk you through the entire process in our signature one-on-one recorded session, explaining the code, the methodology, and the interpretation of the results.
Your Final Deliverable Package
You will receive a complete “Project-in-a-Box” containing everything you need to understand and replicate the analysis:
- A Professional PDF Report: A 5-page summary of the methodology, key findings, and data visualizations, ready for presentation.
- The Annotated Code Script: A fully commented Python Jupyter Notebook/R/SAS that shows you every step of the analysis, from data loading to chart creation.
- Cleaned Data File: A CSV file of the specific data used in the analysis, so you can explore it further on your own.
- The “Co-Pilot” Session Recording: A downloadable video of your personalized walkthrough session, which you can re-watch anytime to reinforce your learning.


Reviews
There are no reviews yet.